EXPAREL uses proprietary multivesicular liposome (pMVL) technology, an advanced drug delivery platform, to extend analgesia1
DESIGNED
to deliver controlled levels of bupivacaine1
COMPOSED
of naturally occurring, biocompatible lipids2-4
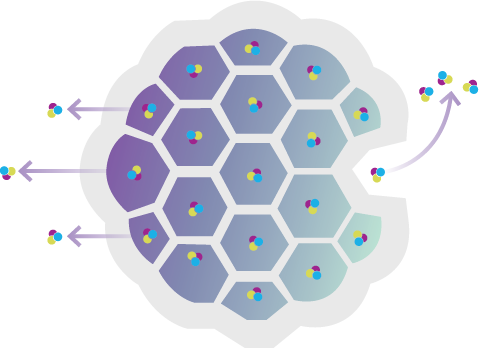
ENCAPSULATES
bupivacaine in a suspension of multivesicular liposomes
RELEASES
bupivacaine over time1

