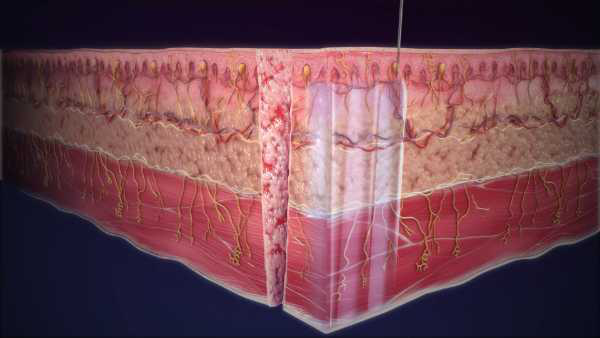
EXPAREL can be administered via infiltration, interscalene brachial plexus nerve block, and fascial plane blocks to provide long-lasting non-opioid pain control
More than 14 million patients have received non-opioid EXPAREL since 2012. EXPAREL works locally at the surgical site and uses the proprietary multivesicular liposome (pMVL) technology, which encapsulates bupivacaine in a suspension of multivesicular liposomes. After injection, bupivacaine is released over time.1,2
The pMVL technology and versatility of administration enables infiltration into the surgical site to produce local analgesia, in the fascial plane to produce regional analgesia as a regional field block or as an interscalene brachial plexus nerve block. EXPAREL may be used across surgical procedures and has demonstrated improved clinical and economic outcomes.
Infiltration
Broad indication across surgical procedures to provide regional analgesia
Field blocks (ie, TAP & PECS)
Use EXPAREL to provide effective regional field or interfascial plane blocks in a range of procedures
TAP, transversus abdominis plane.